Page 12 - full
P. 12
Melting Points
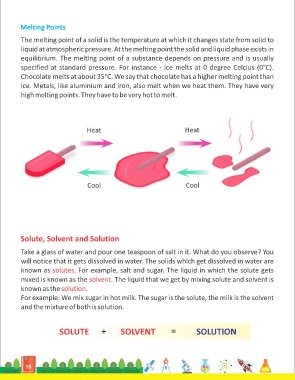
The melting point of a solid is the temperature at which it changes state from solid to
liquid at atmospheric pressure. At the melting point the solid and liquid phase exists in
equilibrium. The melting point of a substance depends on pressure and is usually
specified at standard pressure. For instance - ice melts at 0 degree Celcius (0°C).
Chocolate melts at about 35°C. We say that chocolate has a higher melting point than
ice. Metals, like aluminium and iron, also melt when we heat them. They have very
high melting points. They have to be very hot to melt.
Heat Heat
Cool Cool
Solute, Solvent and Solution
Take a glass of water and pour one teaspoon of salt in it. What do you observe? You
will notice that it gets dissolved in water. The solids which get dissolved in water are
known as solutes. For example, salt and sugar. The liquid in which the solute gets
mixed is known as the solvent. The liquid that we get by mixing solute and solvent is
known as the solution.
For example: We mix sugar in hot milk. The sugar is the solute, the milk is the solvent
and the mixture of both is solution.
SOLUTE + SOLVENT = SOLUTION
16